
Home Electrolysis Experiments - 19/03/18
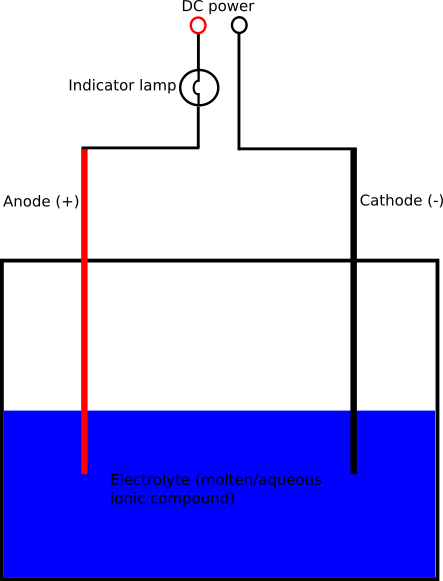
Electrolysis is the decomposition of a chemical compound using electricity. For electrolysis to work, there must be ions present in the compound. Electrolysis of a substance must happen when it is in an aqueous or molten form because, in these states, the ions in the compound are free to move and can thus migrate to their corresponding electrode to be liberated/deposited. In a standard electrolysis cell, two electrodes, the cathode (negative electrode) and the anode (positive electrode) are placed into a liquid which is to be broken down (the electrolyte). When DC current is applied to the electrodes, positive ions in the electrolyte migrate to the negative electrode, gain electrons, and become atoms (the atoms may combine later to form molecules such as hydrogen gas which exists as a diatomic molecule, H2). The negative ions in the electrolyte migrate to the positive electrode where they lose electrons and become atoms.
An excellent example of electrolysis at work is what happens when direct current is passed through impure (conductive) water. Here, the positive hydrogen ions (cations) migrate to the cathode and become hydrogen gas, H2. The negative hydroxide ions (anions) from the water migrate to the anode and are broken down into more water and, interestingly, oxygen gas which, like the hydrogen, leaves the electrode as a stream of bubbles.
Electrolysis is used on an industrial scale for the extraction of aluminium metal. The aluminium ore, bauxite, is dissolved in molten cryolite in order to lower its melting point before being placed in an electrolysis cell where Aluminium metal forms at the negative electrode and oxygen is liberated at the positive electrode. Since the electrodes are made of graphite (carbon), the anode at which the oxygen gas is liberated reacts with the oxygen and is burnt away, thus producing Carbon Dioxide and greatly reducing the lifetime of the anode. The problem of corroding electrodes is quite significant in electrolysis where the electrodes are not meant to be involved in the reaction and can only be mitigated by using very unreactive metals such as platinum.
Below you see a diagram of a typical electrolysis cell:
In order to build a good electrolysis cell, I needed to find a suitable material out of which I could make electrodes. This material needed to be quite corrosion resistant (unreactive) and needed to make minimal mess in the electrolyte when bits broke off it owing to the inevitable corrosion which would eventually happed as I electrolysed water. As mentioned earlier, one could mitigate the problem by using very unreactive electrodes. However, the lower metals in the metal reactivity series are rather expensive so I was thus persuaded to serch for an alternative.
The first material which I used was a pair of steel paperclips. However, the anode at which the oxygen gas was liberated not only corroded with great rapidity but also left a great many brown particles, probably Iron (III) Oxide, in the water as it corroded.
I next tried using a pair of pencils as the cathode and anode because the nibs of the pencils are made largely of graphite which can conduct electricity and thus be used for electrolysing compounds. The cathode to which the positive hydrogen ions migrated was left in perfect condition. The graphite of the anode was eaten away by the oxygen gas which was liberated there. However, this process happened significantly more slowly than with the steel and, what is more, the small particles which broke off the nib of the pencil as it was eaten away barely made the water dirty because there were so few of them. Because of these two notable advantages, I continued the experiments with the pencils. It is important to note that coloured pencils will not work as electrodes because, unlike grey pencils, they are based on a wax nib rather than a graphite-core nib.
Below you see a diagram of my electrolysis cell, using pencils as graphite electrodes:
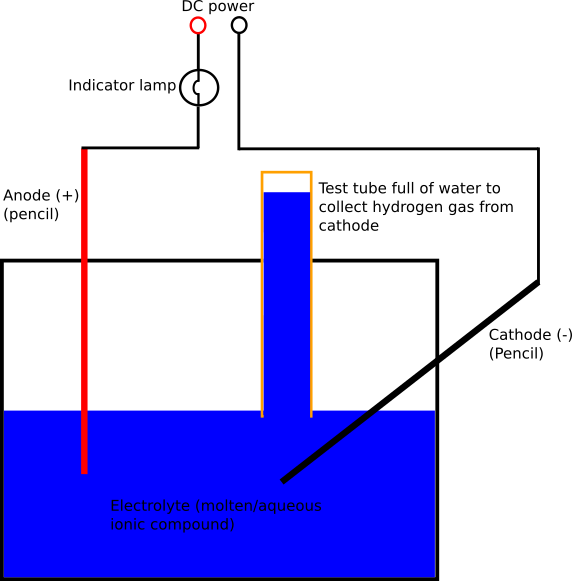
I first started by electrolysing water. This was rather a slow process since water does not naturally ionise to a great extent. Pure (unionised) water does not electrolyse at all because it contains no H+ or OH- ions. Using my aforementioned pencil setup, I placed a test-tube over the cathode to collect the hydrogen gas which would be liberated there. I then applied power from a DC bench power supply at 20 volts. The water on its own drew 20 milliamps. I next added some salt to the water. Salt contains sodium (positive) ions and chloride (negative) ions and conducts electricity when in a molten or aqueous solution. Since I had now created an aqueous solution of sodium chloride, the current draw from the electrolysis cell increased to a few hundred milliamps and more gas was released at the electrodes.
At the cathode, hydrogen gas was released from the water since the ions from sodium metal in the salt were more reactive than hydrogen (if the cation in the aqueous solution is above hydrogen in reactivity series, hydrogen is realeased. If not, the cation is liberated). At the anode, chlorine gas was liberated because it is a halogen (if the anion in the aqueous solution is a halogen, the anion is released. If not, the oxygen is released from the water). The chlorine liberated was a pugnent gas which is toxic so the electrolysis had to be carried out in a well ventilated space (beneath a fume hood).
The solution which was left in the cell was sodium hydroxide because the Na+ ions from the salt combined with the OH- ions from the water to form NaOH, a common base. This is strongly alkaline and has a major use in soaps since it hydrolyses oils and fats. One can test for the presence of a basic compound using PH indicator paper, universal indicator, or a similar indicator substance such as litmus.
One can test for the hydrogen gas by carrying out a test by holding a flame in the cathode-collected test gas. Hydrogen gas should ignite with a loud squeky pop.
Finally, one can also test for oxygen (one of the products of the electrolysis of plain water) by holding a glowing wooden splint in the tube of aquired gas. In the presence of oxygen, the splint will re-ignite.